This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Cannabis and patents? Focus on current trends
On the product side: plants of the Cannabis species produce compounds called cannabinoids. Among these compounds, Δ-9-tetrahydrocannabinol (THC) is known for its powerful psychoactive properties. Cannabidiol (CBD) is also of interest given that it has relaxing effects and relieves anxiety, without the psychotropic effects of THC.
On the legislative side: there have been several recent developments regarding the status of cannabinoids in both France and Europe:
- Published on October 7, 2020, French decree no. 2020-1230 authorizes medical experimentation of cannabinoids in France;
- On November 19, 2020, the European Court of Justice ruled that CBD is not a narcotic product under the 1961 United Nations Convention;
- On December 2, 2020, the United Nations Commission on Narcotic Drugs approved a reclassification of cannabis, previously listed in Schedule IV of the convention which includes the most dangerous drugs.
Cannabinoid-based medicines notably include Sativex (THC + CBD) for patients suffering from multiple sclerosis and Epidyolex (CBD) for the treatment of certain forms of epilepsy. Both are commercialized in several countries; in France, Sativex has a Marketing Authorization, while Epidyolex has a Temporary Use Authorization.
In view of the excitement for these products, the acceleration of scientific research, and legislation which tends to gradually relax the framework for the use of these products, what role do patents play?
First of all, it should be noted that, according to the European Patent Convention (EPC), inventions whose commercial exploitation would be contrary to “ordre public” or morality are not patentable. Nevertheless, it is possible to obtain a patent in Europe for products containing cannabinoids, production processes, and their uses, even though their production or consumption may be prohibited in some states party to the EPC (each state having their own specific reasons).
Secondly, the fact that cannabinoids are naturally occurring substances does not exclude them from patentability in Europe. Indeed, to be patentable, an invention must be new, involve an inventive step, and be industrially applicable. If a naturally occurring substance has been isolated or modified, its patentability can be recognized in Europe.
Numerous patent applications relating to cannabis and its uses have been filed worldwide (approximately 11,500 patent families published to date), including at the European Patent Office (EPO).
The diagram below shows the evolution of the number of patent application publications related to cannabis, filed worldwide (in pink) or filed at the EPO (in red). A clear increase in the number of publications can be seen in 2019 and 2020.
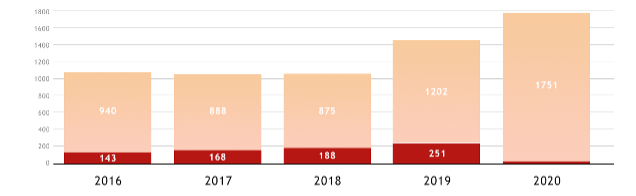
© Questel 2021
Among the top 10 patent applicants at the EPO over the last five years, we note the presence of three companies based in Canada, as well as three American, two Israelian, and two European companies.
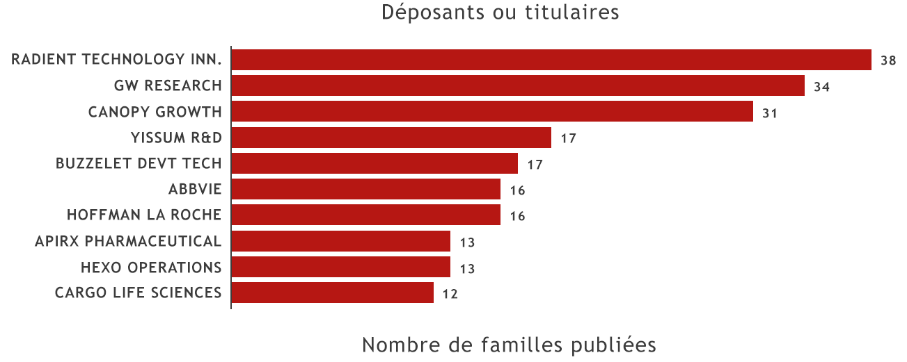
© Questel 2021
With the exception of the pharmaceutical companies ABBVIE, HOFFMAN LA ROCHE and CALICO LIFE SCIENCES, which develop numerous products in addition to those which are cannabis-based, and YISSUM RESEARCH DEVELOPMENT, which is the University of Jerusalem’s valorization department, the main applicants are companies specializing in cannabinoid-based products.
The British company GW RESEARCH commercializes the Sativex and Epidyolex drugs.
The Canadian company RADIENT TECHNOLOGY INNOVATIONS has developed innovative techniques for the extraction and purification of natural cannabinoids. Also based in Canada, CANOPY GROWTH commercializes edible products, beverages, oils, and concentrates for recreational use of cannabis, and HEXO OPERATIONS distributes cannabinoid-based products for both recreational and medicinal uses.
In Israel, BUZZELET DEVELOPMENT & TECHNOLOGIES focuses on cannabinoid purification technologies.
Finally, the American company APIRx PHARMACEUTICAL commercializes GMP grade natural cannabinoids.
Although the main field represented is that of medical cannabis, a significant number of cannabis-associated patents concern food supplements, and thus recreational uses of cannabinoids.
What about cannabis plants? Different varieties of Cannabis sativa exist, varying in their content of certain cannabinoids. These plant varieties, which are not eligible for patent protection in Europe, can nevertheless be protected by a specific title of protection: the Plant Variety Certificate (PVC).
This dynamic developing sector will become more complex in the years to come, especially as legislation in western countries continues to evolve. Undoubtedly, “cannabis innovations” will continue build on the progression observed over the last 5 years.
Actors in this sector must therefore constantly ensure that they are aware of the rights and limits of their activity, taking growing competition into account while also securing their assets.
Our IP Intelligence department, in close association with our patent attorneys, is at your disposal to provide you with the information and reasoned opinions (prior art searches, freedom to operate studies, technology panoramas) necessary for strategic decision-making, to enable you to effectively safeguard your activity, your projects and your titles, while accounting for a particularly dynamic competitive environment.


